The important action in bioburden testing is the collection of samples or Restoration methods of microorganisms from products and medical products. Bioburden testing is highly Employed in pharmaceutical industries, companies of medical units, and cosmetics industries.
Biochemical test or identification by automatic methods may be used for confirmatory identification.
Operating Procedures— Procedures for working the drinking water procedure and performing schedule upkeep and corrective motion should be prepared, they usually should also define the point when action is required. The procedures needs to be very well documented, detail the perform of each work, assign who's chargeable for executing the get the job done, and explain how The work should be to be done. The effectiveness of such procedures ought to be assessed during h2o program validation. Checking Application— Vital high quality attributes and functioning parameters must be documented and monitored. This system may possibly incorporate a combination of in-line sensors or automatic instruments (e.g., for TOC, conductivity, hardness, and chlorine), automatic or handbook documentation of operational parameters (for example move fees or force fall throughout a carbon bed, filter, or RO unit), and laboratory tests (e.g., complete microbial counts). The frequency of sampling, the necessity for evaluating test final results, plus the requirement for initiating corrective action ought to be provided. Sanitization— Dependant upon process structure and the chosen units of Procedure, regime periodic sanitization could possibly be required to preserve the system in a point out of microbial control. Systems for sanitization are explained over. Preventive Maintenance— A preventive upkeep system must be in result. This system should establish what preventive upkeep should be to be performed, the frequency of upkeep function, and how the do the job really should be documented. Modify Control— The mechanical configuration and operating circumstances have to be managed. Proposed variations should be evaluated for his or her impact on The full technique. The necessity to requalify the process following alterations are made must be identified. Pursuing a call to change a water program, the afflicted drawings, manuals, and procedures really should be revised. SAMPLING Factors H2o techniques really should be monitored in a frequency that is certainly ample to make sure that the method is in control and continues to make h2o of acceptable high quality.
Filter the 100ml of water sample and transfer the filter paper very diligently in 100ml Soybean Casein
The inspection need to contemplate the relationship concerning the organisms present in the samples as well as the prospective for the existence of other objectionable disorders. Such as, it is actually reasonable to suppose that if the process would allow E. cloacae to generally be current, it could also allow the existence in the objectionable indicator organism. The microbiologist ought to Examine this opportunity by thinking of this sort of factors as methodology, and The expansion problems of the sample together with other basic factors affiliated with microbiological Examination.
Carry out a negative Handle as test sample, utilizing the picked out pre incubated diluent in place of the test preparation.
Consider ten ml or equivalent volume to 1 g or ml of the products from the above Answer A and transfer to suitable amount of quantity in pre incubated Enterobacteria Enrichment Broth Mossel.
For drug commodities, equally basic safety and effectiveness are crucial. Around the one particular hand, drug safety is decided by whether or not the chemical composition and content material with the drug are Harmless, and on the other hand, drug safety is determined by whether the drug is contaminated by microorganisms. There are many types of microorganisms. Just after contaminating medication, They might decompose the effective ingredients of medications, causing lessened or misplaced efficacy.
Bioburden describes the volume of practical microorganisms existing in an item or over a sterile barrier technique. The bioburden may very well be introduced by a variety of resources like raw products, natural environment, cleaning processes, and manufacturing and assembling components.
The pour plate method is a technique also utilized to isolate and rely feasible microorganisms inside of a provided liquid specimen.
Contact the Baltimore District laboratory for info or questions on these techniques. Vegetation with weighty utilization of those parts of apparatus really should be inspected by people today through the Baltimore District laboratory.
Through the drug microbiological limit test, it is feasible to be aware of whether or not the drug is contaminated and its degree of contamination, to see the supply of the contamination, also to undertake acceptable methods to manage it to be certain the quality of the drug.
People are certainly not obligated to check here make use of precise and maybe archaically produced kinds of analytical drinking water exactly where options with equal or improved high-quality, availability, or analytical general performance may perhaps exist. The regularity and dependability for generating these option analytical waters ought to be confirmed as manufacturing the desired attributes. On top of that, any alternate analytical drinking water has to be evaluated on an application-by-software basis by the person to be sure its suitability. Pursuing is usually a summary of the various types of nonmonographed analytical waters which might be cited while in the USP&#one hundred fifty;NF. Distilled Drinking water— This h2o is made by vaporizing liquid water and condensing it inside of a purer point out. It really is employed principally as being a solvent for reagent preparation, but it is also specified in the execution of other elements of tests, including for rinsing an analyte, transferring a test material as being a slurry, like a calibration conventional or analytical blank, and for test apparatus cleaning. Additionally it is cited because the setting up drinking water for use for building Higher Purity Water. Mainly because none of the cited employs of the water imply a need for a specific purity attribute which will only be derived by distillation, h2o meeting the requirements click here for Purified Drinking water derived by other suggests of purification may very well be equally suitable wherever Distilled H2o is specified. Freshly Distilled H2o— Also referred to as “not long ago distilled water”, it really is created in an identical fashion to Distilled H2o and may be utilized Soon after its generation. This means the need to avoid endotoxin contamination together with almost every other adventitious forms of contamination from your air or containers which could crop up with extended storage. It is used for preparing options for subcutaneous test animal injections along with for any reagent solvent in tests for which there appears to become no specifically large water purity required that might be ascribable to getting “freshly distilled”. While in the “test-animal” use, the phrase “freshly distilled” and its testing use suggest a chemical, endotoxin, and microbiological purity that would be equally pleased by H2o for Injection (however no reference is made to those chemical, endotoxin, or microbial attributes or precise safety from recontamination).
ICH guideline Q4B Annex 4A on analysis and advice of pharmacopoeial texts for use inside the ICH locations on micro enumeration - Move 5
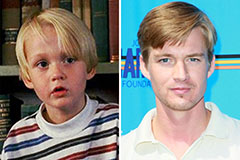 Mason Gamble Then & Now!
Mason Gamble Then & Now!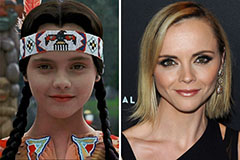 Christina Ricci Then & Now!
Christina Ricci Then & Now! Kenan Thompson Then & Now!
Kenan Thompson Then & Now! Atticus Shaffer Then & Now!
Atticus Shaffer Then & Now! Jeri Ryan Then & Now!
Jeri Ryan Then & Now!